Pyrophosphate is the conjugate base of Pyrophosphoric Acid and is corrosive as well as toxic in nature. Pyrophosphoric Acid is a medium-strong inorganic hygroscopic by nature and is a colourless as well as odourless chemical. Anions, salts, and esters of pyrophosphoric acid are called pyrophosphates. Let us know the chemical properties of Pyrophosphoric Acid.
Properties Of Pyrophosphate
| Chemical formula | H4O7P2 |
| Molecular weight | 177.973 g/mol |
| Chemical Name | Diphosphoric acid, Pyrophosphoric acid
2466-09-3 Phosphonooxyphosphonic acid acide pyrophosphorique |
| Solubility in water | soluble |
| Melting Point | 71.5oC |
| Conjugated base | Pyrophosphate |
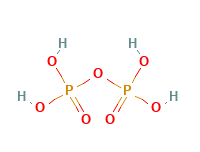
Pyrophosphoric Acid Structural Formula
Pyrophosphoric Acid has been invented by Mr. Clarke of Glasgow in the year 1827. Pyrophosphoric Acid is an acyclic phosphorus acid anhydride obtained by condensation of two molecules of phosphoric acid. The structural formula of Pyrophosphoric Acid is as shown in the figure below.
For more such interesting information along with videos, subscribe BYJU’S!!
Comments